Investor Information
Executive Summary
There are more than four million patients suffering from end-stage kidney disease (ESKD) worldwide. Most of these patients will require hemodialysis for renal replacement therapy. It is imperative for patients to have a well-functioning vascular access for optimal hemodialysis therapy. According to the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, an autogenous arteriovenous fistula (AVF) is the preferred vascular access for hemodialysis. Unfortunately, approximately 60% of AVFs develop a venous stenosis at one year, thus preventing optimal hemodialysis from being administered. Currently, there are no therapies that can prevent the formation of AVF stenosis. Our groundbreaking innovation decreases venous stenosis formation by coating the AVF with an anti-inflammatory, which leads to less blockage of the blood vessel. This reduces the need for costly invasive procedures such as angioplasty that are otherwise needed to maintain the patency and function of AVFs.
We are actively seeking investment to support our first in-human studies. Funds will be used to perform a first in human study including biomanufacturing the drug and obtaining FDA IND approval with IRB approval.
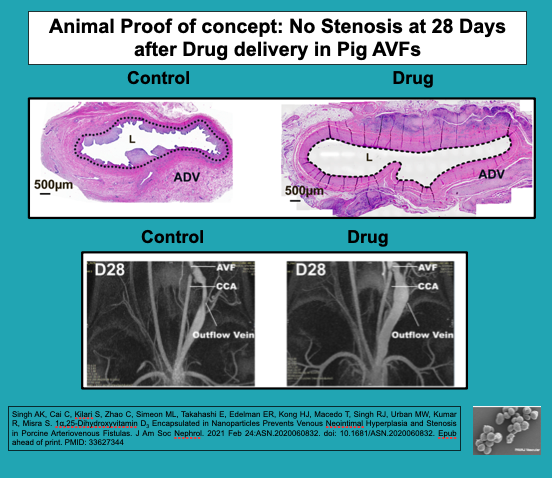
Our large animal data show that there are no safety issues with our drug delivery platform. Moreover, there is a significant improvement in the lumen vessel area and blood flow. We are seeking $10M to complete GLP testing (if needed), finalize a GMP drug manufacturing contract, and conduct phase 1 human studies. These studies will be performed at a single center to evaluate the outcomes for safety and efficacy in 40 patients. The resulting data will allow us to raise Series B and C funding to perform a larger pivotal study. To complete this work, we will need three years.
Product Description:
After over a decade of research funded by the National Institutes of Health (NIH), we have created a groundbreaking drug delivery application. This can be used intraoperatively or in the interventional catheterization laboratory, as demonstrated in our three publications and two patents (US10098897B2 and WO2016011397A1). This innovation has been tested in mice and pigs, and it has been shown to prevent vascular stenosis that results after the placement of an arteriovenous fistula (AVF) used for hemodialysis. It also prevents restenosis after angioplasty of AVF stenosis.
Currently, there are no therapies available that can prevent venous stenosis. Once a stenosis develops, it is treated with angioplasty or, more recently, paclitaxel-coated balloons (which have no reimbursement code). Our drug is called, 1,25 NPs, which is administered locally to the artery and vein at the time of AVF creation or after angioplasty, thereby reducing stenosis formation.
The Science:
Over the past twelve years, we have tested many different drug and biologic therapies aimed at preventing venous stenosis formation. The current drug (1,25 NP) demonstrated a significant reduction in venous stenosis associated with AVF in both pigs and mice. 1,25 NP was created by encapsulating 1,25 (OH2)D3Vit D3 (active vitamin D3) in nanoparticles composed of polylactic-co-glycolic acid (PLGA) and loaded in hydrogel (1,25 NP) for sustained drug release after delivery.
Scope of the Problem Being Addressed:
In the United States, there are more than 800,000 patients with a diagnosis of ESKD. Each year, approximately 40,000 AVFs are placed in the US. In addition, more than 200,000 angioplasty procedures are performed in patients with vascular access malfunction. The cost of loss of AVF patency per year is $17,000 per AVF, or $408 million across 24,000 cases of loss of AVF patency.
Based on our pig data, we believe 1,25 NP will reduce stenosis by 50% at 6 months, provide a savings of $8.5K (2022) to the health system per case, and reduce morbidity associated with invasive procedures. Reimbursement payments are made by CMS and Medicare/Medicaid.
In aggregate, for reducing stenosis after placement of an AVF and after PTA, the US market size is estimated to be $6.4B. The worldwide AVF market is significantly larger, with ≈ 4M patients with ESKD.
Additional Licensed Technologies:
Other cardiovascular applications of this technology include PTFE AV grafts (US: 10K/year), coronary artery bypass grafts (US: 500K/year), arterial bypass grafts for peripheral arterial disease (PAD: 100K/year), coronary artery stents (US: 1M/year), PAD stents and angioplasty (US: 300K/year), and venous stents (US: 80K/year). In sum, this is nearly 2M procedures at a potential US market of $20 billion. The worldwide market for these therapies is much larger.